Beverage Innovation Through Newamstar Aseptic Systems
In today’s competitive beverage market, producers face mounting pressure to deliver products with extended shelf life, preserved nutritional value, and impeccable safety standards—all without relying on preservatives. Aseptic filling technology has emerged as the definitive solution to these challenges, revolutionizing how beverages are processed, packaged, and distributed globally.
At the forefront of this technological revolution stands Newamstar, a pioneer in PET bottle aseptic filling technology. Since its inception, Newamstar has continuously pushed the boundaries of aseptic filling innovation, evolving from basic systems to today’s highly sophisticated, fully integrated solutions.
A Trusted Partner in Beverage Production
As a trusted partner to beverage enterprises worldwide, Newamstar has consistently upheld its mission to “help clients build ideal factories.” Through continuous research, innovation, and unwavering dedication, the company has become a guardian of beverage production excellence. From the early stages of traditional wet sterilization technology to the widespread application of advanced dry aseptic technology, Newamstar has remained deeply involved, pioneering innovations that have established it as the founding architect of China’s PET bottle aseptic filling industry.
The company continues to enhance its aseptic blow-fill-seal solutions with new capabilities and competitive advantages, helping clients achieve digitalized, intelligent, and environmentally sustainable beverage production across diverse operational scenarios.
The Evolution of Newamstar’s Aseptic Filling Technology
Newamstar’s journey in aseptic filling technology spans decades of innovation, each milestone introducing significant advancements that have collectively transformed the industry landscape.
1999: Pioneering Beginnings
Successfully developed China’s first aseptic filling machine, establishing the foundation for continuous innovation and technological advancement in the domestic market.
2005: International Recognition
Signed a cooperation agreement with Japan’s Otsuka for four PET bottle aseptic filling lines, becoming the first Chinese manufacturer to export aseptic filling production lines to developed countries.
2008: Strategic Partnerships
Established a strategic partnership with Dali Group, delivering three aseptic filling production lines initially. To date, Newamstar has provided over thirty wet and dry aseptic filling production lines to the Dali Group.
Early 2010s: Third & Fourth Generations
These generations marked revolutionary advancements with fully automated CIP/SIP systems, enhanced HEPA filtration, sophisticated isolator technology, and improved product flow paths. Fourth-generation machines achieved speeds of up to 36,000 bottles per hour while maintaining the highest standards of aseptic integrity.
2015: Seventh Generation Launch
First released Newamstar’s globally leading seventh-generation aseptic filling technology. This breakthrough innovation employed electron beam sterilization technology to replace traditional sterilization methods, providing customers with lower operating costs, superior environmental performance, and greater production flexibility.
Recent Years: Continuous Innovation
Newamstar has successively launched a series of new technologies and products, including dry aseptic carbonated blow-fill-seal systems, integrated dry aseptic blow-fill-seal solutions, and third-generation electron beam aseptic blow-fill-seal technology. These innovations have gained widespread recognition and become powerful enablers of sustainable development in the industry.
“Looking back on the journey of aseptic technology development and the numerous challenges overcome, Newamstar continues to break records with its competitive spirit and determination. The company has become Asia’s only supplier of blow-fill-seal production lines to successfully commercialize all three major aseptic technologies: PAA wet sterilization, hydrogen peroxide dry sterilization, and electron beam sterilization. It is also the first Chinese equipment supplier to secure contracts for over 100 aseptic lines!”
Understanding Aseptic Filling Technology
Aseptic filling represents a sophisticated process where commercially sterilized products are packaged in pre-sterilized containers within a sterile environment. This method eliminates harmful microorganisms without compromising product quality, enabling ambient storage without refrigeration for extended periods—typically 6-12 months.
Unlike traditional hot-fill or retort processes that apply heat after packaging, aseptic technology sterilizes the product and packaging separately before bringing them together in a controlled environment. This critical distinction preserves flavor profiles, nutritional components, and textural properties that would otherwise degrade under conventional thermal processing.
What exactly is aseptic filling technology, and how does it differ from other filling methods?
Aseptic filling technology involves separately sterilizing the product and packaging materials, then bringing them together in a sterile environment to create a commercially sterile product that remains shelf-stable without refrigeration or preservatives.
This differs fundamentally from other methods:
- Hot-fill sterilizes the container using the heat from the hot product itself (typically 85-95°C), requiring heat-resistant packaging and resulting in some quality degradation from extended heat exposure.
- Cold-fill with preservatives uses chemical preservatives to prevent microbial growth but avoids the quality impact of heat, though consumers increasingly reject preservative use.
- Tunnel pasteurization fills products at ambient temperature, then pasteurizes the sealed containers in a heating tunnel, requiring heavy packaging to withstand thermal stress.
- ESL (Extended Shelf Life) uses milder thermal treatment with clean filling conditions for moderate shelf life extension, but typically requires refrigeration.
Newamstar’s aseptic technology achieves commercial sterility while minimizing thermal impact on the product, enabling ambient distribution without preservatives and allowing lightweight packaging designs impossible with hot-fill or retort processes.
Key Benefits of Aseptic Filling Technology
- Extended Shelf Life: Products remain stable at ambient temperatures for 6-12 months without preservatives
- Superior Nutrient Retention: Gentle processing preserves vitamins, enzymes, and functional ingredients
- Enhanced Flavor Profiles: Minimal thermal impact maintains authentic taste and sensory characteristics
- Reduced Energy Consumption: Up to 30% less energy compared to conventional technologies
- Expanded Distribution Options: Ambient storage eliminates cold chain requirements
- Increased Production Efficiency: Integrated systems maximize output while minimizing waste
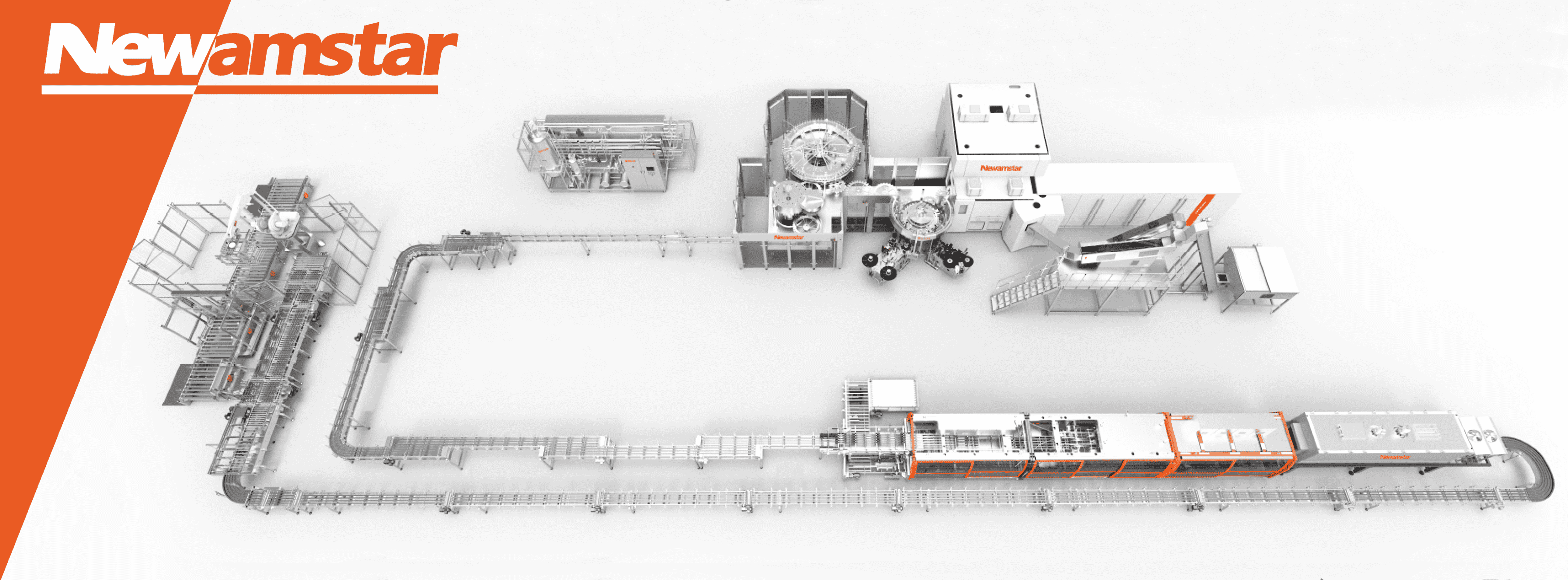
Core Components of Newamstar’s Aseptic Filling Systems
Newamstar’s aseptic filling systems comprise several integrated subsystems working together:
1. Multi-Method Sterilization Technologies
Newamstar integrates multiple sterilization methods including PAA-based wet sterilization, hydrogen peroxide dry sterilization, and electron beam sterilization. This flexibility allows producers to select the optimal approach based on specific product characteristics and production requirements, ensuring maximum microbiological safety while preserving product integrity.
2. Product Treatment System
Complete systems including Ultra-High Temperature (UHT) processing modules, homogenization systems, deaeration systems for oxygen-sensitive products, specialized treatment modules for specific formulations, and aseptic surge tanks maintaining sterility between processes.
3. Filling Technology
Proprietary low-shear filling valves maintain product integrity by minimizing turbulence during the filling process. Non-contact filling valves prevent cross-contamination, while electromagnetic or mass-flow metering ensures precise volume control. Specialized filling systems accommodate different product characteristics with inert gas management systems and aseptic transfer systems between sterile zones.
4. Clean Room Environment Management
The entire operation takes place within a controlled Class 100 (ISO 5) environment with HEPA filtration, positive pressure systems, and sophisticated airflow management. This hermetically sealed ecosystem includes positive pressure barriers, laminar airflow systems, environmental monitoring systems, and personnel and material access controls to prevent contamination throughout the production process.
5. Intelligent Control Systems
Advanced monitoring capabilities operate through intuitive interfaces, providing real-time data on critical production parameters. Integrated automation controls all subsystems, while process monitoring ensures critical parameter maintenance. Validation systems document sterility assurance, with recipe management for different product formulations and comprehensive traceability systems documenting production history.
Applications Across Beverage Categories
The versatility of Newamstar’s aseptic filling technology extends across the entire spectrum of liquid products, with specialized configurations optimized for different beverage categories:
Plant-Based Beverages
Specialized systems address the unique challenges of plant proteins, preventing sedimentation and maintaining homogeneity throughout shelf life. The gentle processing preserves delicate flavor compounds and nutritional elements found in plant-based alternatives.
Dairy Products
Advanced technologies preserve milk proteins and prevent fat separation while ensuring extended ambient stability without refrigeration. This represents a significant breakthrough for dairy products that traditionally required cold chain distribution.
Fruit Juices
Low-shear processing preserves natural enzymes and antioxidants, maintaining fresh flavor profiles and nutritional integrity. Specialized solutions for pulp management ensure consistent product quality from the first bottle to the last.
Tea Beverages
Specialized sterilization prevents degradation of delicate flavor compounds and functional tea extracts throughout shelf life, preserving both taste and health benefits that consumers expect from premium tea products.
Coconut Water
Precise thermal control prevents degradation of coconut water’s delicate flavor profile and nutritional compounds, delivering a shelf-stable product that maintains the authentic taste and benefits of fresh coconut water.
Functional Beverages
Specially designed systems ensure the stability and efficacy of bioactive compounds, vitamins, and probiotics in functional beverages, opening new possibilities for the fast-growing health and wellness segment.
Coffee Beverages
Advanced processing techniques maintain the distinctive aroma compounds and flavor notes of specialty coffee products, allowing manufacturers to deliver premium ready-to-drink coffee with extended shelf stability and no quality compromise.
Sports & Energy Drinks
Precision-engineered systems ensure the preservation of performance-enhancing compounds and electrolyte profiles in sports nutrition products, delivering consistent efficacy and bioavailability throughout the product’s extended shelf life.
Carbonated Beverages
Newamstar’s dry aseptic carbonated blow-fill-seal systems maintain carbonation levels while ensuring product sterility, offering manufacturers the ability to produce preservative-free carbonated beverages with extended shelf life and superior taste profiles.
Operational Considerations
What regulatory standards do Newamstar’s systems comply with?
Newamstar’s aseptic systems are designed to meet global regulatory requirements:
Food Safety Standards:
- FDA requirements for aseptic processing
- European Union hygiene regulations
- FSMA (Food Safety Modernization Act) compliance
- China FDA requirements
- Codex Alimentarius guidelines
Quality System Standards:
- ISO 9001 quality management system
- FSSC 22000 food safety system certification
- GMP (Good Manufacturing Practice) requirements
- HACCP (Hazard Analysis Critical Control Point) principles
- ISO 14159 hygiene requirements
Newamstar’s regulatory affairs team assists customers with regulatory documentation and approval processes, particularly for products exported to international markets with varying requirements.
Economic and Business Considerations
What is the typical return on investment (ROI) for Newamstar’s aseptic systems?
ROI varies based on application, but typical financial metrics include:
Payback Period:
- Average range: 2-4 years
- Premium product applications: Often 1.5-2.5 years
- High-volume commodity applications: Typically 3-5 years
Return Drivers:
- Packaging material reduction (typically 20-30% vs. hot-fill)
- Energy savings (25-40% vs. alternative technologies)
- Extended distribution range without cold chain
- Premium pricing opportunities through quality enhancement
- Labor efficiency through automation
- Waste reduction and yield improvement
Newamstar provides detailed ROI analysis tools during the evaluation process, incorporating customer-specific parameters for accurate financial modeling.
How does the total cost of ownership compare to alternative technologies?
Total Cost of Ownership (TCO) analysis typically reveals:
Capital Investment:
- Higher initial investment than hot-fill or preservative-based systems
- Lower than HTST plus cold-chain distribution when considering total system costs
- Comparable to or lower than retort systems for similar capacity
Operational Costs:
- Lower packaging material costs (20-30% savings vs. hot-fill)
- Reduced energy consumption (25-40% savings vs. hot-fill)
- Eliminated cold chain costs for ambient distribution
- Lower labor requirements through automation
- Reduced product waste through precision filling and extended shelf life
Comprehensive TCO analysis tools are available to evaluate specific application scenarios with customer-specific parameters.
What financing and payment options are available?
Newamstar offers flexible acquisition approaches:
Traditional Purchase:
- Standard progress payments based on project milestones
- Customized payment schedules aligned with project phases
- Volume-based discounting for multiple line purchases
- Technology bundle pricing for integrated solutions
Financing Options:
- Equipment leasing through financial partners
- Capital equipment loans with competitive terms
- Export financing through international development banks
- Vendor financing programs for qualified customers
Newamstar’s finance team works with customers to develop optimal acquisition strategies aligned with capital budgeting and financial objectives.
Implementation and Practical Considerations
How should we prepare our facility for aseptic technology implementation?
Optimal preparation includes:
Facility Requirements:
- Clean room or controlled environment areas
- Appropriate utility services at required capacities
- Adequate floor space with proper load-bearing capacity
- Environmental controls (temperature, humidity, pressure)
- Material and personnel flow design for aseptic operations
Pre-Implementation Activities:
- Comprehensive site assessment and gap analysis
- Utility capacity evaluation and enhancement if needed
- Personnel training and skill development
- Quality system preparation for aseptic operations
- Supply chain readiness for new packaging materials
Newamstar provides detailed facility requirement specifications and pre-implementation guidance, including site assessment services to identify specific preparation needs.
What are common implementation challenges and how are they addressed?
Typical challenges and mitigation approaches include:
Technical Challenges:
- Challenge: Product formulation compatibility with aseptic processing
Solution: Product development support and pilot testing services - Challenge: Validation complexity for aseptic processes
Solution: Structured validation protocols and regulatory expertise - Challenge: Integration with existing production systems
Solution: Comprehensive integration planning and interface management
Operational Challenges:
- Challenge: Personnel skill development for aseptic operations
Solution: Tiered training programs and competency certification - Challenge: Quality system adaptation for new technology
Solution: Quality system gap analysis and development support - Challenge: Production scheduling during implementation
Solution: Phased installation planning minimizing disruption
Newamstar’s project methodology incorporates structured risk management and mitigation planning, addressing these common challenges proactively through the implementation process.
Global Impact and Industry Recognition
Throughout its evolutionary journey, Newamstar has successfully delivered over 2,300 production lines to customers across more than 100 countries and regions worldwide. The company’s aseptic filling technology has been embraced by international beverage giants including Coca-Cola, PepsiCo, Danone, and Nestlé, as well as leading domestic brands like Master Kong, Wahaha, and Nongfu Spring.
As a testament to its technical excellence, Newamstar has received numerous industry accolades and holds over 500 invention patents, many specifically related to aseptic filling innovations. This recognition reflects not only the company’s technological capabilities but also its commitment to driving industry advancement.
Key Achievements in Aseptic Filling Technology
Future Vision and Continuous Innovation
Looking to the future, Newamstar continues to refine and enhance its aseptic filling technology with a clear vision of sustainable, efficient, and intelligent production. The company is actively developing solutions that align with global trends in sustainability, digitalization, and consumer preferences for healthier, more natural products.
What production speeds can Newamstar’s aseptic systems achieve?
Newamstar offers aseptic systems across a range of production capacities:
- Standard commercial ranges: 6,000 to 80,000 bottles per hour
- Most common implementations: 24,000 to 48,000 bottles per hour
- High-speed systems: Up to 80,000 bottles per hour for water and CSD products
- Specialized high-capacity systems: Up to 108,000 bottles per hour (currently the highest capacity in Asia)
Systems are typically specified with 10-15% capacity margin above current requirements to accommodate future growth, with modular designs enabling capacity expansion in many cases.
What maintenance requirements should we anticipate?
Maintenance for Newamstar’s aseptic systems follows a structured approach:
Routine Maintenance (Daily/Weekly):
- Visual inspections of critical components
- Sensor calibration verification
- Sterile filter integrity testing
- Seal and gasket inspections
- Minor lubrication and adjustment tasks
Scheduled Maintenance (Monthly/Quarterly):
- Comprehensive preventive maintenance tasks
- Wear component replacement based on running hours
- Control system diagnostics
- Validation of critical parameters
- CIP/SIP system performance verification
Modern systems incorporate predictive maintenance capabilities using vibration analysis, thermal imaging, and performance trending to identify developing issues before failures occur, typically reducing unplanned downtime by 30-50% compared to traditional calendar-based maintenance.
Quality and Regulatory Aspects
How does Newamstar ensure aseptic integrity and product safety?
Product safety is ensured through multiple complementary approaches:
Design for Safety:
- Hygienic design principles eliminating contamination risk points
- Material selection meeting FDA, EHEDG, and 3-A standards
- Fail-safe systems defaulting to product protection
- Redundant critical systems preventing single-point failures
- Isolation technology preventing environmental contamination
Validation Methodology:
- Installation Qualification verifying proper system setup
- Operational Qualification confirming function within specifications
- Performance Qualification demonstrating consistent sterility assurance
- Media fills validating the complete aseptic process
- Challenge testing confirming system robustness
These complementary approaches create multiple safety barriers, ensuring consistent product protection even under challenging conditions.


