Evolution of Newamstar Innovative Aseptic Filling
For beverage manufacturers considering aseptic filling technology, the decision-making process involves numerous questions spanning technical capabilities, operational requirements, economic considerations, and implementation approaches. This comprehensive FAQ addresses the most common inquiries about Newamstar’s aseptic filling technology, providing clear, detailed answers to support informed evaluation and implementation planning.
The questions are organized into logical categories, allowing readers to focus on aspects most relevant to their specific needs, whether they’re in the initial exploration phase or actively planning implementation. Each answer provides practical insights based on Newamstar’s extensive experience across global markets and diverse beverage applications.
Technology Fundamentals
What exactly is aseptic filling technology, and how does it differ from other filling methods?
Aseptic filling technology involves separately sterilizing the product and packaging materials, then bringing them together in a sterile environment to create a commercially sterile product that remains shelf-stable without refrigeration or preservatives.
This differs fundamentally from other methods:
- Hot-fill sterilizes the container using the heat from the hot product itself (typically 85-95°C), requiring heat-resistant packaging and resulting in some quality degradation from extended heat exposure.
- Cold-fill with preservatives uses chemical preservatives to prevent microbial growth but avoids the quality impact of heat, though consumers increasingly reject preservative use.
- Tunnel pasteurization fills products at ambient temperature, then pasteurizes the sealed containers in a heating tunnel, requiring heavy packaging to withstand thermal stress.
- ESL (Extended Shelf Life) uses milder thermal treatment with clean filling conditions for moderate shelf life extension, but typically requires refrigeration.
Newamstar’s aseptic technology achieves commercial sterility while minimizing thermal impact on the product, enabling ambient distribution without preservatives and allowing lightweight packaging designs impossible with hot-fill or retort processes.
Quality and Regulatory Aspects
How does Newamstar ensure aseptic integrity and product safety?
Product safety is ensured through multiple complementary approaches:
Design for Safety:
- Hygienic design principles eliminating contamination risk points
- Material selection meeting FDA, EHEDG, and 3-A standards
- Fail-safe systems defaulting to product protection
- Redundant critical systems preventing single-point failures
- Isolation technology preventing environmental contamination
Validation Methodology:
- Installation Qualification verifying proper system setup
- Operational Qualification confirming function within specifications
- Performance Qualification demonstrating consistent sterility assurance
- Media fills validating the complete aseptic process
- Challenge testing confirming system robustness
These complementary approaches create multiple safety barriers, ensuring consistent product protection even under challenging conditions.
What regulatory standards do Newamstar’s systems comply with?
Newamstar’s aseptic systems are designed to meet global regulatory requirements:
Food Safety Standards:
- FDA requirements for aseptic processing
- European Union hygiene regulations
- FSMA (Food Safety Modernization Act) compliance
- China FDA requirements
- Codex Alimentarius guidelines
Quality System Standards:
- ISO 9001 quality management system
- FSSC 22000 food safety system certification
- GMP (Good Manufacturing Practice) requirements
- HACCP (Hazard Analysis Critical Control Point) principles
- ISO 14159 hygiene requirements
Newamstar’s regulatory affairs team assists customers with regulatory documentation and approval processes, particularly for products exported to international markets with varying requirements.
“The evolution of Newamstar’s aseptic filling technology reflects not just the growth of a single company but the advancement of an entire industry—establishing new standards for safety, efficiency, and sustainability in beverage production worldwide.”
Economic and Business Considerations
What is the typical return on investment (ROI) for Newamstar’s aseptic systems?
ROI varies based on application, but typical financial metrics include:
Payback Period:
- Average range: 2-4 years
- Premium product applications: Often 1.5-2.5 years
- High-volume commodity applications: Typically 3-5 years
Return Drivers:
- Packaging material reduction (typically 20-30% vs. hot-fill)
- Energy savings (25-40% vs. alternative technologies)
- Extended distribution range without cold chain
- Premium pricing opportunities through quality enhancement
- Labor efficiency through automation
- Waste reduction and yield improvement
Newamstar provides detailed ROI analysis tools during the evaluation process, incorporating customer-specific parameters for accurate financial modeling.
How does the total cost of ownership compare to alternative technologies?
Total Cost of Ownership (TCO) analysis typically reveals:
Capital Investment:
- Higher initial investment than hot-fill or preservative-based systems
- Lower than HTST plus cold-chain distribution when considering total system costs
- Comparable to or lower than retort systems for similar capacity
Operational Costs:
- Lower packaging material costs (20-30% savings vs. hot-fill)
- Reduced energy consumption (25-40% savings vs. hot-fill)
- Eliminated cold chain costs for ambient distribution
- Lower labor requirements through automation
- Reduced product waste through precision filling and extended shelf life
Comprehensive TCO analysis tools are available to evaluate specific application scenarios with customer-specific parameters.
Implementation and Practical Considerations
How should we prepare our facility for aseptic technology implementation?
Optimal preparation includes:
Facility Requirements:
- Clean room or controlled environment areas
- Appropriate utility services at required capacities
- Adequate floor space with proper load-bearing capacity
- Environmental controls (temperature, humidity, pressure)
- Material and personnel flow design for aseptic operations
Pre-Implementation Activities:
- Comprehensive site assessment and gap analysis
- Utility capacity evaluation and enhancement if needed
- Personnel training and skill development
- Quality system preparation for aseptic operations
- Supply chain readiness for new packaging materials
Newamstar provides detailed facility requirement specifications and pre-implementation guidance, including site assessment services to identify specific preparation needs.
What are common implementation challenges and how are they addressed?
Typical challenges and mitigation approaches include:
Technical Challenges:
- Challenge: Product formulation compatibility with aseptic processing
Solution: Product development support and pilot testing services - Challenge: Validation complexity for aseptic processes
Solution: Structured validation protocols and regulatory expertise - Challenge: Integration with existing production systems
Solution: Comprehensive integration planning and interface management
Operational Challenges:
- Challenge: Personnel skill development for aseptic operations
Solution: Tiered training programs and competency certification - Challenge: Quality system adaptation for new technology
Solution: Quality system gap analysis and development support - Challenge: Production scheduling during implementation
Solution: Phased installation planning minimizing disruption
Newamstar’s project methodology incorporates structured risk management and mitigation planning, addressing these common challenges proactively through the implementation process.
Global Impact and Industry Recognition
Throughout its evolutionary journey, Newamstar has successfully delivered over 2,300 production lines to customers across more than 100 countries and regions worldwide. The company’s aseptic filling technology has been embraced by international beverage giants including Coca-Cola, PepsiCo, Danone, and Nestlé, as well as leading domestic brands like Master Kong, Wahaha, and Nongfu Spring.
As a testament to its technical excellence, Newamstar has received numerous industry accolades and holds over 500 invention patents, many specifically related to aseptic filling innovations. This recognition reflects not only the company’s technological capabilities but also its commitment to driving industry advancement.
Key Achievements in Aseptic Filling Technology
Conclusion: Making an Informed Decision
Aseptic filling technology represents a significant advancement in beverage production capability, offering compelling advantages in product quality, operational efficiency, and market opportunity. Newamstar’s systems deliver these benefits through advanced technology, comprehensive support, and continuous innovation.
For beverage manufacturers navigating changing consumer preferences, sustainability requirements, and competitive market pressures, aseptic technology provides a powerful platform for successful product development and market expansion. By understanding the capabilities, requirements, and implementation approaches outlined in this FAQ, manufacturers can make informed decisions about how this technology aligns with their specific business objectives and operational needs.
Whether you’re in the initial exploration phase or actively planning implementation, Newamstar’s technical and commercial teams are available to provide additional information, answer specific questions, and support your evaluation process with data-driven analysis tailored to your unique requirements.
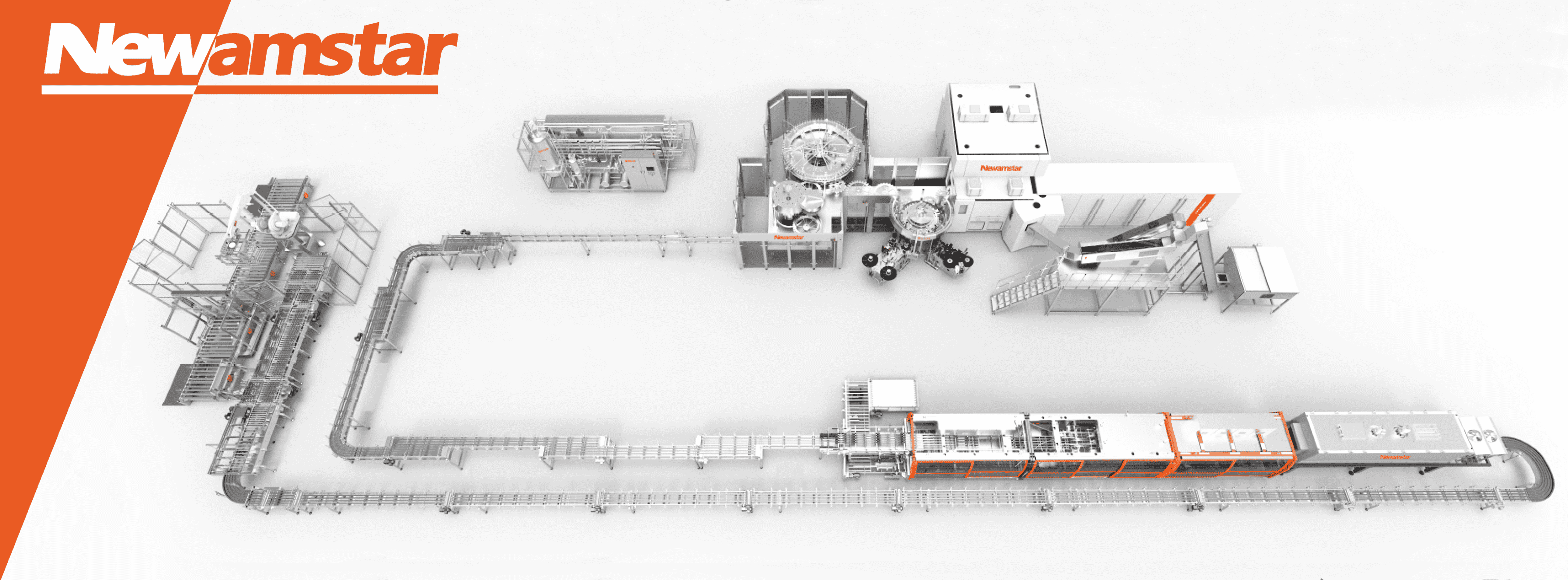
What are the core components of Newamstar’s aseptic filling systems?
Newamstar’s aseptic filling systems comprise several integrated subsystems working together:
1. Product Treatment System: Typically including:
- Ultra-High Temperature (UHT) processing module
- Homogenization systems (where required)
- Deaeration systems for oxygen-sensitive products
- Specialized treatment modules for specific formulations
- Aseptic surge tanks maintaining sterility between processes
2. Sterilization Systems:
- Product sterilization via thermal or alternative technologies
- Container sterilization typically using hydrogen peroxide vapor, radiation, or heat
- Cap/closure sterilization with specialized technologies
- Environmental sterilization maintaining aseptic filling zone
- CIP/SIP systems for equipment sterilization
3. Filling Technology:
- Non-contact filling valves preventing cross-contamination
- Electromagnetic or mass-flow metering for precise volume control
- Specialized filling systems for different product characteristics
- Inert gas management systems (where required)
- Aseptic transfer systems between sterile zones
4. Environmental Control:
- HEPA filtration systems maintaining air quality
- Positive pressure barriers preventing contamination ingress
- Laminar airflow systems minimizing particulate movement
- Environmental monitoring systems verifying conditions
- Personnel and material access controls
5. Control Systems:
- Integrated automation controlling all subsystems
- Process monitoring ensuring critical parameter maintenance
- Validation systems documenting sterility assurance
- Recipe management for different product formulations
- Traceability systems documenting production history
These components work as an integrated system to maintain product sterility throughout processing and packaging, with each element critical to overall system effectiveness.
Key Benefits of Aseptic Filling Technology
- Extended Shelf Life: Products remain stable at ambient temperatures for 6-12 months without preservatives
- Superior Nutrient Retention: Gentle processing preserves vitamins, enzymes, and functional ingredients
- Enhanced Flavor Profiles: Minimal thermal impact maintains authentic taste and sensory characteristics
- Reduced Energy Consumption: Up to 30% less energy compared to conventional technologies
- Expanded Distribution Options: Ambient storage eliminates cold chain requirements
- Increased Production Efficiency: Integrated systems maximize output while minimizing waste
What product types and packaging formats can Newamstar’s aseptic technology handle?
Product Categories:
- Low-acid beverages (pH > 4.6) including dairy, plant-based alternatives, protein drinks
- High-acid beverages including juices, teas, coffee drinks, flavored waters
- Pulp-containing products with particles up to 10mm in size
- Viscous products up to 150 cP
- Carbonated products with specialized aseptic carbonation systems
- Hot-fill compatible products for hybrid processing approaches
Packaging Formats:
- PET bottles from 200ml to 5L in various designs
- Custom container designs with specialized neck finishes
- Multi-layer barrier bottles for oxygen-sensitive products
- Lightweight containers impossible with hot-fill technology
- Various closure styles including screw caps, sport caps, and custom designs
Specialized configurations are available for unique product characteristics or packaging requirements, with system customization based on specific application needs.
The Evolution of Newamstar’s Aseptic Filling Technology
Newamstar’s journey in aseptic filling technology spans multiple generations of innovation, each introducing significant advancements that have collectively transformed the industry landscape.
Early 2000s: First Generation
Newamstar’s initial entry into aseptic technology featured basic sterilization processes with speeds of approximately 12,000 bottles per hour. These pioneering systems laid the foundation for future innovations while meeting the growing market demand for safer, longer-lasting beverage products.
Mid-2000s: Second Generation
The second generation brought more sophisticated sterilization techniques and improved automation, doubling production speeds to 24,000 bottles per hour while enhancing reliability and operational consistency.
Late 2000s – Early 2010s: Third & Fourth Generations
These generations marked revolutionary advancements with fully automated CIP/SIP systems, enhanced HEPA filtration, sophisticated isolator technology, and improved product flow paths. Fourth-generation machines achieved speeds of up to 36,000 bottles per hour while maintaining the highest standards of aseptic integrity.
Mid-2010s: Fifth & Sixth Generations
The transition toward intelligent manufacturing principles characterized these generations, featuring integrated digital control platforms, advanced vision inspection systems, comprehensive data analytics, and improved energy efficiency. Sixth-generation equipment reached impressive speeds of 48,000 bottles per hour while actually reducing energy consumption.
Present Day: Seventh Generation
Today’s seventh-generation systems represent the pinnacle of Newamstar’s innovation journey, integrating multiple sterilization technologies, maintaining 100-level cleanliness standards, and achieving filling accuracy of ±0.2% at speeds reaching an extraordinary 80,000 bottles per hour.
Operational Considerations
What production speeds can Newamstar’s aseptic systems achieve?
Newamstar offers aseptic systems across a range of production capacities:
- Standard commercial ranges: 6,000 to 80,000 bottles per hour
- Most common implementations: 24,000 to 48,000 bottles per hour
- High-speed systems: Up to 80,000 bottles per hour for water and CSD products
- Specialized high-capacity systems: Up to 108,000 bottles per hour (currently the highest capacity in Asia)
Systems are typically specified with 10-15% capacity margin above current requirements to accommodate future growth, with modular designs enabling capacity expansion in many cases.
What utility requirements do these systems have?
Utility requirements vary based on system configuration and capacity, but typical requirements include:
Electrical:
- 380-480V, 50/60Hz, three-phase power
- Connected load typically 300-800kW depending on capacity
- Actual consumption approximately 60-70% of connected load
- UPS backup for critical control systems
Steam:
- Culinary-grade steam for product heating
- Industrial steam for CIP/SIP systems
- Typical consumption: 500-1,200 kg/hour depending on system size
- Pressure requirements: 6-10 bar
Detailed utility specifications are provided during project planning, with options for utility optimization based on local conditions and costs.
What maintenance requirements should we anticipate?
Maintenance for Newamstar’s aseptic systems follows a structured approach:
Routine Maintenance (Daily/Weekly):
- Visual inspections of critical components
- Sensor calibration verification
- Sterile filter integrity testing
- Seal and gasket inspections
- Minor lubrication and adjustment tasks
Scheduled Maintenance (Monthly/Quarterly):
- Comprehensive preventive maintenance tasks
- Wear component replacement based on running hours
- Control system diagnostics
- Validation of critical parameters
- CIP/SIP system performance verification
Modern systems incorporate predictive maintenance capabilities using vibration analysis, thermal imaging, and performance trending to identify developing issues before failures occur, typically reducing unplanned downtime by 30-50% compared to traditional calendar-based maintenance.
How long does implementation typically take?
Implementation timelines vary based on project scope, but typical timeframes include:
Project Phases:
- Initial specification and design: 2-3 months
- Equipment manufacturing: 4-6 months
- Factory acceptance testing: 2-3 weeks
- Shipping and delivery: 1-2 months
- Installation: 4-8 weeks
- Commissioning: 2-4 weeks
- Validation: 2-6 weeks
- Production ramp-up: 1-3 months
Total timeline from contract to commercial production:
- Standard projects: 12-16 months
- Fast-track projects: 9-12 months
- Complex implementations: 16-20 months
Core Components of Modern Aseptic Systems
Newamstar’s seventh-generation aseptic filling systems represent the current pinnacle of liquid packaging technology, integrating multiple advanced technologies to achieve unprecedented levels of performance, safety, and efficiency.
1. Multi-Method Sterilization Technologies
Newamstar integrates multiple sterilization methods including PAA-based wet sterilization, hydrogen peroxide dry sterilization, and electron beam sterilization. This flexibility allows producers to select the optimal approach based on specific product characteristics and production requirements, ensuring maximum microbiological safety while preserving product integrity.
2. Intelligent Control Systems
Advanced monitoring capabilities operate through intuitive interfaces, providing real-time data on critical production parameters. Predictive maintenance algorithms identify potential issues before they impact production, while comprehensive data logging ensures complete traceability and quality assurance throughout the manufacturing process.
3. Clean Room Environment Management
The entire operation takes place within a controlled Class 100 (ISO 5) environment with HEPA filtration, positive pressure systems, and sophisticated airflow management. This hermetically sealed ecosystem prevents contamination throughout the production process, from preform sterilization to final packaging.
4. Specialized Filling Valve Technology
Proprietary low-shear filling valves maintain product integrity by minimizing turbulence during the filling process. This technology is particularly critical for sensitive products like juices with pulp, dairy alternatives, and protein-enriched beverages, where molecular structure and suspension stability must be preserved.
Preform
Blow Molding
Filling
Capping
Labeling
Packaging
Frequently Asked Questions
What types of beverages can be processed on Newamstar’s aseptic filling lines?
Newamstar’s aseptic filling systems can process a wide range of beverages including water, juices, teas, dairy products, plant-based milks, protein drinks, sports beverages, and other liquid food products. Our systems are designed to handle both high-acid (pH below 4.6) and low-acid (pH above 4.6) products.
What is the shelf life of products filled on Newamstar’s aseptic systems?
Products filled on our aseptic systems typically achieve shelf lives of 6-12 months without refrigeration, depending on the specific product characteristics and packaging materials used. Some products can achieve even longer shelf life with appropriate formulation and packaging.
What bottle sizes can be handled on Newamstar’s aseptic filling lines?
Our aseptic systems can handle PET bottles ranging from 250ml to 2.0L, with specific capacity ranges varying by system configuration. Custom solutions for other sizes may be available upon consultation.
How does Newamstar ensure the sterility of the packaging process?
Newamstar employs multiple sterilization technologies including PAA wet sterilization, hydrogen peroxide dry sterilization, and electron beam sterilization. The entire process occurs within a controlled cleanroom environment with HEPA-filtered air, positive pressure, and continuous monitoring of critical control points.
What is the typical installation time for an aseptic filling line?
Installation time varies depending on line complexity and capacity, but typically ranges from 3-6 months from equipment delivery to production qualification. Our experienced project management team works closely with customers to minimize downtime and ensure smooth implementation.
Does Newamstar provide training for operating personnel?
Yes, comprehensive training programs are included with all Newamstar aseptic systems. Training covers operation, maintenance, troubleshooting, and quality control procedures. Both on-site and factory training options are available.
Global Aseptic Technology Presence
With over 2,300 liquid product production lines successfully implemented across more than 100 countries and regions globally, Newamstar has established a worldwide footprint of excellence. Our technology adaptability ensures optimal performance across diverse operating environments, climate conditions, and regulatory frameworks.
Regional Solution Adaptations
- Tropical Climate Solutions: Enhanced environmental control systems for high-humidity regions
- High-Altitude Installations: Specialized pressure control systems for elevated locations
- Cold Climate Implementations: Thermal protection systems for low-temperature environments
- Challenging Water Quality Regions: Enhanced water treatment capabilities
Global Regulatory Compliance
- FDA (Food and Drug Administration) requirements
- EHEDG (European Hygienic Engineering & Design Group) guidelines
- 3-A Sanitary Standards
- China NMPA regulations
- FSSC 22000 food safety management system requirements
- ISO 14159 (Hygiene requirements for machinery design)
Global Aseptic Solutions Provider
Aseptic Installations
Production Lines
Countries & Regions
Sterilization Technologies
From fruit juices to dairy products, and from tea beverages to plant-based alternatives, Newamstar has successfully implemented aseptic solutions across a diverse range of applications worldwide.
Our expertise spans the full spectrum of liquid product categories, with tailored solutions that address the unique challenges of each product type while maintaining the highest standards of quality and safety.
Complete Aseptic Production Ecosystem
Maximize your investment with Newamstar’s comprehensive aseptic production ecosystem. Our integrated solutions work in perfect harmony to deliver exceptional efficiency, product quality, and operational excellence.
Transform Your Beverage Production
Contact our aseptic technology experts today to discuss your specific production requirements and discover how Newamstar’s advanced aseptic filling systems can elevate your manufacturing capabilities.


