Understanding 6D Sterilization in Aseptic Filling Systems
In the world of aseptic processing, sterilization effectiveness is measured not in simple percentages but in logarithmic reductions of microbial populations. Among these measurements, the “6D” standard has emerged as a critical benchmark for safety and quality in aseptic filling systems. Newamstar, a global leader in liquid packaging solutions, has pioneered technologies that consistently achieve this gold standard for low-acid products. But what exactly does “6D” mean, and why is it so important for beverage safety and quality?
Defining the 6D Standard
The “D” in the 6D standard refers to the decimal reduction time, or the time required to kill 90% of a specific microorganism’s population under specific conditions. The number preceding “D” indicates how many of these decimal reductions are achieved—in this case, six.
A 6D reduction means reducing the initial microbial population by six logarithmic cycles, or in simpler terms, achieving a 99.9999% reduction in the target microorganism. This means that for every million microorganisms initially present, only one would statistically remain after treatment.
“For low-acid products (pH > 4.6), which are more susceptible to pathogenic bacterial growth, industry standards and regulatory requirements typically specify a minimum 6D reduction for commercial sterility.”
The Mathematical Perspective
To better understand the 6D standard, consider the mathematical expression:
Where:
- N₀ is the initial number of microorganisms
- N is the number of surviving microorganisms after treatment
- 10-6 represents a reduction of 6 logarithmic cycles
If we start with 1,000,000 microorganisms (106), a 6D treatment would reduce this to just 1 surviving microorganism. This reduction level provides a statistically significant safety margin for commercial sterility.
Newamstar’s Multi-barrier Approach to 6D Achievement
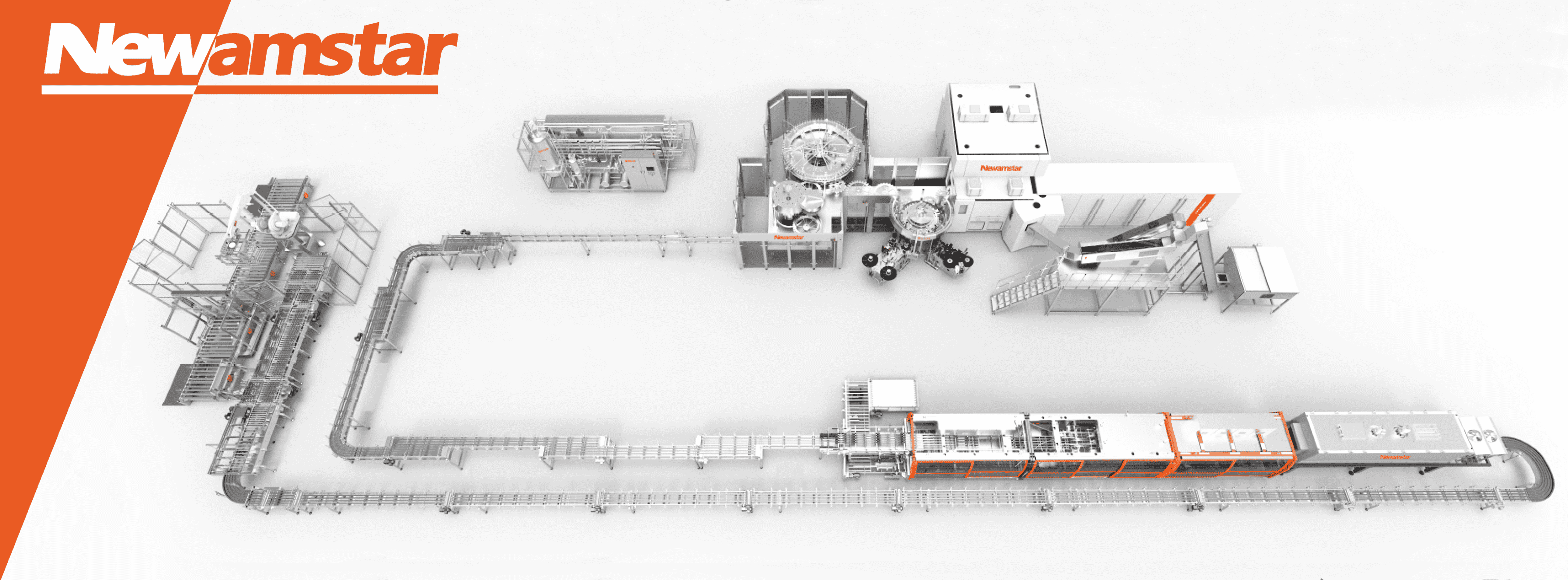
Newamstar’s aseptic filling systems employ a sophisticated multi-barrier approach to achieve and often exceed the 6D standard. This approach recognizes that relying on a single sterilization method may leave vulnerabilities in the process. Instead, Newamstar integrates multiple sterilization technologies, each contributing to the overall microbial reduction.
1. Product Sterilization
The primary method for product sterilization in Newamstar’s systems is Ultra-High Temperature (UHT) treatment, which typically achieves well beyond 6D reduction for most pathogens. The system allows for precise temperature control with options including direct steam injection achieving rapid heating to 135-150°C, indirect heat exchangers for more gentle product treatment, hold tubes ensuring proper dwell time at sterilization temperatures, and rapid cooling to preserve product quality attributes.
2. Package Sterilization
Achieving 6D reduction in packaging materials requires specialized approaches depending on the material type. Newamstar’s systems incorporate multiple technologies including hydrogen peroxide vapor (H₂O₂) treatment followed by hot sterile air drying for PET bottles, specialized sterilization chambers using concentrated hydrogen peroxide and UV irradiation for bottle caps, and electron beam technology providing precise sterilization for complex packaging components.
3. Environment Sterilization
The environment where aseptic filling takes place must maintain exceptional cleanliness. Newamstar’s systems create an ISO Class 5 (Class 100) environment through HEPA filtration systems with 99.997% efficiency for particles 0.3μm and larger, positive pressure differentials preventing ingress of outside contaminants, laminar airflow design minimizing turbulence and particle movement, UV irradiation of air supplies, and specialized cleaning protocols for all surfaces within the aseptic zone.
Validation and Verification of 6D Achievement
Achieving the 6D standard is meaningless without proper validation and ongoing verification. Newamstar implements comprehensive protocols to ensure consistent performance:
Biovalidation
Using resistant test organisms (typically Bacillus atrophaeus or Geobacillus stearothermophilus) to challenge the system under controlled conditions.
Challenge Testing
Performed under worst-case conditions to ensure the system maintains adequate sterilization even at operational extremes.
Media Fills
Simulating actual production conditions using growth media instead of product to detect any potential contamination issues.
Statistical Analysis
Rigorous mathematical evaluation of results confirming 6D reduction achievement across all critical control points.
Continuous Verification
During normal operation, multiple verification measures ensure ongoing compliance with the 6D standard:
Ongoing Verification Measures
- Real-time monitoring of critical process parameters
- Regular microbiological sampling and testing
- Automated alarms for deviations from critical limits
- Scheduled revalidation procedures at defined intervals
- Media fills performed according to regulatory requirements
Technological Innovations Enhancing 6D Performance
Newamstar’s commitment to research and development has resulted in several innovations that enhance the reliability and efficiency of 6D sterilization:
Advanced PAA Sterilization
Peracetic acid formulations developed specifically for Newamstar’s systems provide enhanced effectiveness against resistant microorganisms while reducing chemical consumption.
Pulsed Light Technology
High-intensity pulsed light technology for surface sterilization delivers additional microbial reduction, particularly effective against fungi and spores that may show resistance to chemical treatments.
Integrated Electron Beam
Integration of electron beam technology for packaging sterilization represents a significant advancement, allowing precise dosing control and elimination of chemical residues.
Smart Sterilization Control
AI-driven control systems continuously optimize sterilization parameters based on real-time product and environmental conditions, ensuring consistent 6D achievement.
Practical Implications of 6D Achievement
The consistent achievement of the 6D standard in Newamstar’s aseptic filling systems translates to several practical benefits for manufacturers:
Extended Shelf Life
Products filled on Newamstar’s 6D-validated systems typically achieve ambient shelf life of 6-12 months, enabling broader market reach and reduced waste.
Cold Chain Independence
Unlike products requiring refrigeration, aseptically filled products can be distributed through conventional supply chains, dramatically reducing logistics costs.
Clean Label Opportunities
The high safety margin provided by 6D sterilization eliminates the need for chemical preservatives, supporting clean label trends and consumer preferences.
Regulatory Compliance
Newamstar’s systems meet or exceed regulatory requirements in all major markets, including FDA, EU, and CFDA standards for aseptic processing.
“The 6D sterilization standard represents the foundation of safety in modern aseptic filling technology. Newamstar’s multi-barrier approach, sophisticated validation protocols, and continuous technological innovation ensure that this critical standard is not merely met but consistently exceeded.”
Conclusion
For beverage manufacturers, the 6D standard translates to confidence in product safety, quality, and regulatory compliance. As consumer preferences continue to evolve toward clean-label, preservative-free products with extended shelf life, the importance of reliable 6D sterilization technology becomes increasingly apparent.
Newamstar’s unwavering commitment to the 6D standard places it at the forefront of aseptic technology providers, delivering systems that protect both consumers and brand reputation through scientifically validated safety protocols and cutting-edge sterilization technology.
Global Impact and Industry Recognition
Throughout its evolutionary journey, Newamstar has successfully delivered over 2,300 production lines to customers across more than 100 countries and regions worldwide. The company’s aseptic filling technology has been embraced by international beverage giants including Coca-Cola, PepsiCo, Danone, and Nestlé, as well as leading domestic brands like Master Kong, Wahaha, and Nongfu Spring.
As a testament to its technical excellence, Newamstar has received numerous industry accolades and holds over 500 invention patents, many specifically related to aseptic filling innovations. This recognition reflects not only the company’s technological capabilities but also its commitment to driving industry advancement.